Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
Showing Original Post only (View all)The Effect of Fugitive Hydrogen Emissions on Extreme Global Heating. [View all]
Last edited Sun Apr 13, 2025, 11:34 AM - Edit history (3)
The paper I'll discuss in this post, containing as a first sentence a nonsense statement but worth reading anyway, is this one:
Quantification of Hydrogen Emission Rates Using Downwind Plume Characterization Techniques Ahmad Momeni, John D. Albertson, Scott Herndon, Conner Daube, David Nelson, Joseph Robert Roscioli, Joanne Shorter, Elizabeth Lunny, Rick Wehr, Greeshma Gadikota, Yuanfeng Cui, and Tianyi Sun Environmental Science & Technology 2025 59 (12), 6016-6024
The paper is open sourced, there is no reason to excerpt it extensively.
The first sentence in the paper is this one, again, a nonsense statement:
Hydrogen (H2) plays a key role in current plans to decarbonize parts of industry and energy systems.
It is a nonsense statement because overwhelmingly, on any scale that matters, hydrogen is manufactured by the steam reformation of dangerous fossil fuels accompanied by the destruction of exergy. Making hydrogen makes carbon emissions worse, not better.
EST: Chinese Hydrogen Production Is Making Climate Change Worse.
A Giant Climate Lie: When they're selling hydrogen, what they're really selling is fossil fuels.
What is interesting however, is that the release of fugitive hydrogen apparently also makes the atmospheric chemistry by a direct mechanism of which I was until coming across this paper, I was unaware.
(1,2) However, studies show that H2 emissions have an indirect warming power that is about 37 times that of carbon dioxide (CO2) pound-for-pound over 20 years and 12 times that over 100 years. (3−6) The added H2 in the atmosphere depletes the hydroxyl radical (OH), which is a primary sink for methane, and thus contributes to warming through a longer lifetime of atmospheric methane. It also produces tropospheric ozone and stratospheric water vapor─all three are short-lived greenhouse gases. (6) Because of this indirect warming effect, recent studies indicate that high H2 emissions along the value chain can severely undermine the intended climate benefits of H2 solutions, especially in the near and medium terms. (1,4,5,7) However, there are currently few studies and limited knowledge on the magnitude and intensity of H2 emissions from today’s value chain. (8) One primary reason is the unavailability of fast and precise sensor technology for accurately measuring fugitive or operational H2 emissions at the facility level. This limited availability of empirical measurements has forced attempts to quantify value chain and component-level emissions of H2 to rely on assumptions that are obtained from proxies, theoretical models, and simulations, which contain high levels of uncertainty. (8) These estimates are on questionable footing without empirical validation, as H2 molecules are far smaller than other greenhouse gases such as methane, and hence they are logically more prone to fugitive emissions. (9) To date, there are no established measurement and quantification methods for H2 emissions at the facility level.
Therefore, this study aims to fill this knowledge gap by (1) deploying a state-of-the-art fast and precise H2 sensor technology in a controlled-release field testing, (2) applying tracer flux ratio (TFR) methodology (10−12) to assess the sensor’s capability in enabling the quantification of H2 emission rates through comeasurements and comparison with other well-studied trace gases, and (3) evaluating the applicability of Bayesian-based plume model inversion for H2 emission quantification using the prototype sensor in the absence of tracers.
Therefore, this study aims to fill this knowledge gap by (1) deploying a state-of-the-art fast and precise H2 sensor technology in a controlled-release field testing, (2) applying tracer flux ratio (TFR) methodology (10−12) to assess the sensor’s capability in enabling the quantification of H2 emission rates through comeasurements and comparison with other well-studied trace gases, and (3) evaluating the applicability of Bayesian-based plume model inversion for H2 emission quantification using the prototype sensor in the absence of tracers.
I have placed that previously unknown mechanism in bold above; the bold is mine.
A long term implication of an industrial "hydrogen economy" which happily is a bunch of bullshit advertised by the fossil fuel industry along with carbon sequestration and similar bullshit, and thus will never become a big deal, is, theoretically at least, implied by the statistical theory of gases.
It goes like this:
Helium is the second most common element in the universe, but is rare on Earth, all of it arising from the decay of uranium and thorium (and their daughter elements in their decay chain). The reason for this is the low atomic weight of helium.
The average kinetic energy of a gas molecule is determined by the Maxwell-Boltzmann distribution, and is equal to 3/2kT, where T is the temperature on the thermodynamic temperature (Kelvin) scale.
Thus the average velocity of a gas molecule can be determined by the equation 1/2 Mv2 = 3/2kT, where M is the mass of a molecule or atom in a gas and v is the average velocity.
If we solve this equation for v we have (3kT/M)1/2 = v
At 300K, roughly room temperature, solving using the average molecular weight of H2 gas, 2.02 Daltons, which translates into 3.3211 X 10-27 kg and Boltzmann's constant, 1.38 X 10-23 JK-1, we find that an average hydrogen molecule is traveling at 1933 ms-1, or 1.933 km-s-1.
The escape velocity of the Earth is 11.2 km-s-1. However the speed determined above is the average speed of hydrogen molecules at 300K. The Maxwell-Boltzmann distribution actually is, as the name implies, a distribution, with a roughly Poisson type shape.
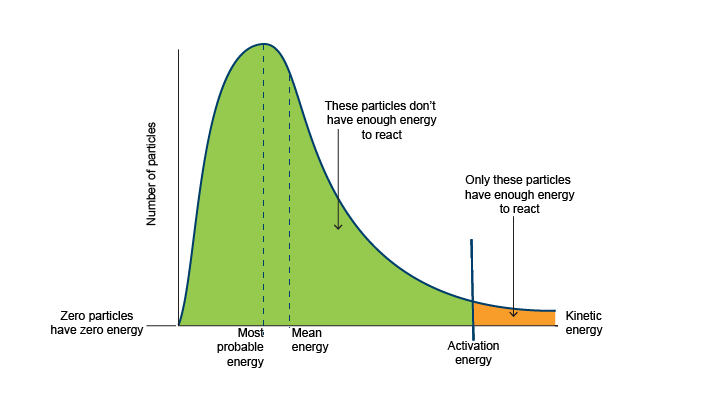
It turns out that a significant portion of the hydrogen molecules actually actually exceed 11.2 km-s-1
This explains why hydrogen and helium, respectively the first and second most common elements in the Universe are rare in Earth's atmosphere.
A nice video of the subject with a Russian accented scientist explaining the point is here:
Hydrogen released into the atmosphere is eventually boiled off into space, as is helium. I'm not sure that this would be a big deal in a putative hydrogen economy, which is actually a stupid idea that happily will never happen, but over a long term, it might matter.
Have a nice Sunday.
6 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
Re first sentence, "plays a role" does not express a preference for policy; is different from "should play a role"
Bernardo de La Paz
Apr 2025
#1
Hydrogen can be formed for useful purposes by electrolysing water with energy from nuclear power
Bernardo de La Paz
Apr 2025
#2