Environment & Energy
Showing Original Post only (View all)Zeolite Catalysts for the Low Temperature Production of the Wonder Fuel DME. [View all]
Dimethyl ether (DME) is a wonder fuel. A gas, at ambient temperatures, (its boiling temperature is -24 °C), it has a critical temperature of 126.95°C means that it can be liquified at temperatures above the boiling point of water. (It has thus been used as a refrigerant.) It is generally nontoxic, albeit with slight anesthetic effects at high concentrations, is easily removed from water, and has an atmospheric half-life of about 5 days.
These properties mean that it can substitute fairly easily for all uses currently using propane or other forms of LPG.
It is well known that DME can run diesel engines without producing significant air pollution, owing to its lack of a carbon-carbon bond, which greatly reduces the production of particulates.
In 2011, the late great Nobel Laureate George Olah proposed a closed carbon cycle via DME (or methanol) to address the on going and accelerating tragedy of extreme global heating with which we now live:
Anthropogenic Chemical Carbon Cycle for a Sustainable Future George A. Olah, G. K. Surya Prakash, and Alain Goeppert Journal of the American Chemical Society 2011 133 (33), 12881-12898.
DME can be made in two steps via the hydrogenation of carbon dioxide to methanol, following dehydration of the methanol to DME, or, alternatively, by the hydrogenation of carbon dioxide in a single step. The following paper refers to the former process:
Tuning RHO Zeolite Crystallization Time and Precursors for Stable and Improved Methanol Conversion to Dimethyl Ether Compared to Conventional Catalysts Alireza Lotfollahzade Moghaddam, Mohammad Ghavipour, Jan Kopyscinski, and Melanie Jane Hazlett, ACS Sustainable Chemistry & Engineering 2024 12 (19), 7478-7486
From the introductory text, covering much of what I said above:
There are two routes through which DME can be produced: the direct method (one step) and the indirect method (two steps). (7) In the direct method, methanol is synthesized and dehydrated simultaneously in a single reactor containing a bifunctional catalyst. On the other hand, the two-step route, which has been of primary interest to industries, is performed in two separate reactors loaded with proper catalysts. (8) A Cu-ZnO-Al2O3 catalyst with Zr, Mn, La, and Ce promoters is commonly used in CO/CO2 hydrogenation reactions to reach methanol, (9−11) whereas solid acid catalysts such as γ-Al2O3, zeolites, heteropoly acid catalysts (HPAs), aluminum phosphate, and ion exchange resins have been investigated for methanol dehydration. (12−14) The reactions taking place in the indirect and direct processes are listed in eqs 1–4:

The notable thing about DME is that it is a "drop in" replacement for dangerous natural gas, dangerous LPG, and dangerous diesel fuel. No major infrastructure changes are needed to use it, other than changing the chemistry of some seals in diesel engines. This is very different than all of the very stupid, dangerous, and tiresome crap one hears from the fossil fuel industry and its advertisers about hydrogen. The key issue with the production of DME is the source of the hydrogen to make it. As is the case with hydrogen itself, the source matters. DME is only sustainable where the primary energy source is nuclear energy, preferably avoiding the wasteful intermediate of electricity - a thermodynamically degraded form of energy - but rather via thermochemical cycles such as the sulfur iodine cycle. Conducted in a process intensification approach, where the production of thermodynamically degraded electricity is a side product, rather than the main product of nuclear heat, this is an effective approach to closing the industrial anthropogenic carbon cycle, which is, as of now, not a "cycle" at all: The atmosphere is a waste dump.
The authors note that industrially, where DME is produced as a propellant to replace CFC's in spray cans, the dehydration reaction is conducted over a form of γ-Al2O3. The problem with these catalysts is their stability; they often require replacement.
RHO zeolite is a specific member of the class of zeolites - generally caged structures of aluminum and silicon oxides - that the authors investigate for the dehydration reaction.
They conclude that the stability of the RHO zeolite catalyst addresses the stability problem with these catalysts.
A figure from the paper:
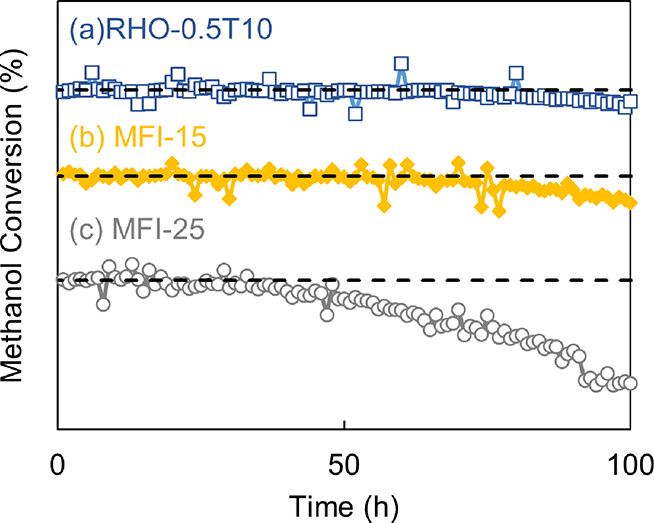
The caption:
The figure lacks numbers in the "percent talk" on the ordinate. This is probably a production error; perhaps it will be corrected.
There are many thousands of papers written on DME production; I'm not sure that this one is among the best, but I thought I'd refer to it anyway, if nothing more than to point out that nuclear generated DME - the paper does not refer to hydrogen sources - is a route to the closed carbon cycle Olah envisioned before his death.
I trust you're having a nice weekend. Happy "fall back" for those experiencing it. Let's make sure that on Tuesday, our nation does not fall back into the hands of a senile corrupt criminal uneducated racist thug, and that instead, we welcome our first, hopefully among many others to follow, female chief executive.