Environment & Energy
Related: About this forumRadioactive Cesium for Chemical Production, Hydrogen Peroxide from Seawater..
The paper I'll discuss in this post is this one: Bandgap Regulation Engineering of MXene Ti3C2 by Radioactive Cesium Uptake and Sustainable Photocatalytic Production of H2O2 in Real Seawater Hongfei Mei, Jinsong Peng, Haiyan Song, and Chunxia Chen Industrial & Engineering Chemistry Research 2025 64 (45), 21386-21403.
I have been working in recent months on some ideas to leave with my son with my ideas for uses of fission product cesium from used nuclear fuel, exploiting its neutronic properties, specifically the relatively high capture cross section of the only stable non-radioactive isotope of cesium 133Cs, the idea being to transform it into the highly radioactive isotope 134Cs. (134Cs does not form directly in used nuclear fuel since 134Xe is a stable isotope.)
It has always struck me that cesium isotopes, in particular the most famous 137Cs are very useful materials, and indeed they do have some limited industrial uses already, albeit relatively small.
This paper refers to a new one to me, using it to make the valuable liquid, hydrogen peroxide, which is currently made using a comparatively dirty process using anthraquinone, an aromatic organic molecule.
Mxenes are layered materials formed by partial dissolution of compounds called "Max Phases." These were popularized by Michel Barsoum of Drexel University, with whom I once had a very pleasant dinner.
From the paper's introduction:
Significant in-depth exploration is also underway in the field of photocatalysis. Ling et al. developed an octonary high-entropy oxide (HEO) (Ti/V/Cr/Nb/Mo/W/Al/Cu) that integrates eight earth-abundant metal elements into a single rutile phase. This material achieves full-spectrum visible-light absorption enabled by its high-density oxygen vacancies and chemical complexity, thus facilitating efficient H2O2 production in the absence of any cocatalysts or sacrificial agents. (19) Wang et al. constructed a resorcinol–formaldehyde resin/MgIn2S4 S-scheme heterojunction (RF/MIS). The optimized material achieved a H2O2 production rate of 892.6 μmol·L–1·h–1. This breakthrough provides a new strategy for developing organic photocatalysts with a broad-spectrum response. (20) Yang et al. synthesized heteroaromatic covalent organic frameworks (TTT-COFs), enhancing stability and extending the π-conjugated system by introducing sulfur atoms. The resulting material combines high chemical stability with a reinforced donor–acceptor structure. It achieved a production rate of 29.9 mmol·g–1·h–1 in a system containing 10 vol % benzyl alcohol as a sacrificial agent. (21)
Although light, water, and O2 are adopted in this method, most of the reported systems still encounter the problems of dependence on organic proton–electron donors, pure O2 gas, and freshwater to increase the H2O2 productivity, which increases process complexity and costs. Seawater is one of the most abundant resources on Earth. Photocatalytic H2O2 production from seawater can reduce costs, and the as-prepared H2O2 can potentially be used in desalination, disinfection, and marine ecosystem protection. There have been a certain number of reports on the photoproduction of H2O2 in seawater. (22−29) However, more efficient photocatalysts for H2O2 production are in urgent need of being developed to solve the issues of dependence on organic proton–electron donors and pure O2, as well as the influence of competitive ions in seawater.
Radioactive nuclide wastewater contains uranium, cesium, strontium, barium, etc. Among them, radioactive 137Cs has a high fission yield and is highly radioactive. (30) During the release of 137Cs, a large amount of radiation is generated with a half-life of up to 30 years, which greatly affects the ecological environment and human health. Many technologies, such as ion exchange, adsorption, filtration, chemical precipitation, and solvent extraction, have been developed for the treatment of nuclear wastewater containing cesium. (31−34) Among them, the adsorption method has the advantages of feasibility, high efficiency, and low cost, and is widely used in removing Cs+ from wastewater. As a member of the absorbent materials, it is reported that a wide variety of nuclear element adsorbent materials have been documented, including oxides, (35) metal–organic frameworks (MOFs), (36) graphene, (37) zeolites, (38) etc. MXene Ti3C2 and its derivatives have been frequently reported in the elimination of radioactive nuclides (e.g., Sr2+, Cs+) from nuclear wastewater. (39−45) MXene Ti3C2 is one of the famous transition metal carbides, possessing a two-dimensional (2D) layered structure, a high specific surface area, and sufficient adsorption sites...
I won't have a lot of time, regrettably, to cover this wonderful paper, so nice to see, fully.
For flavor, a few figures from the text.
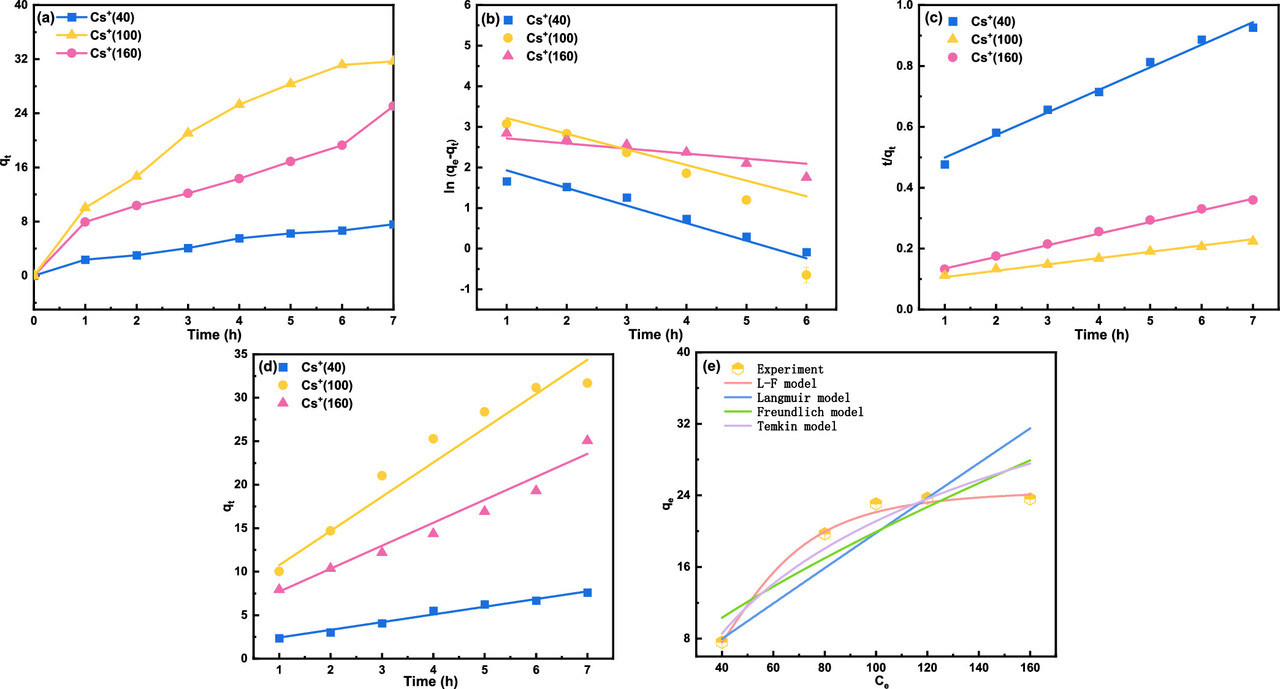
The caption:
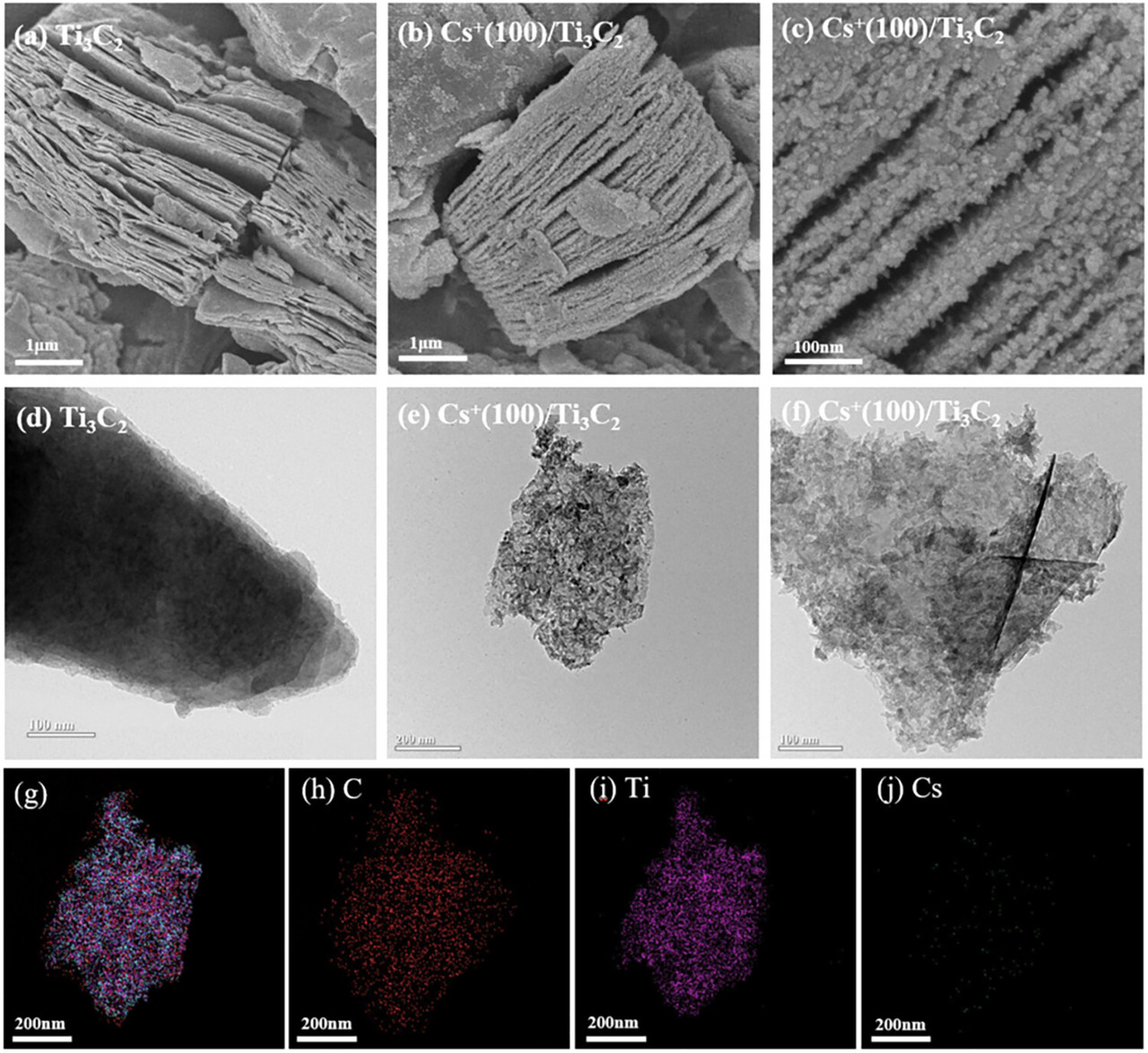
The caption:
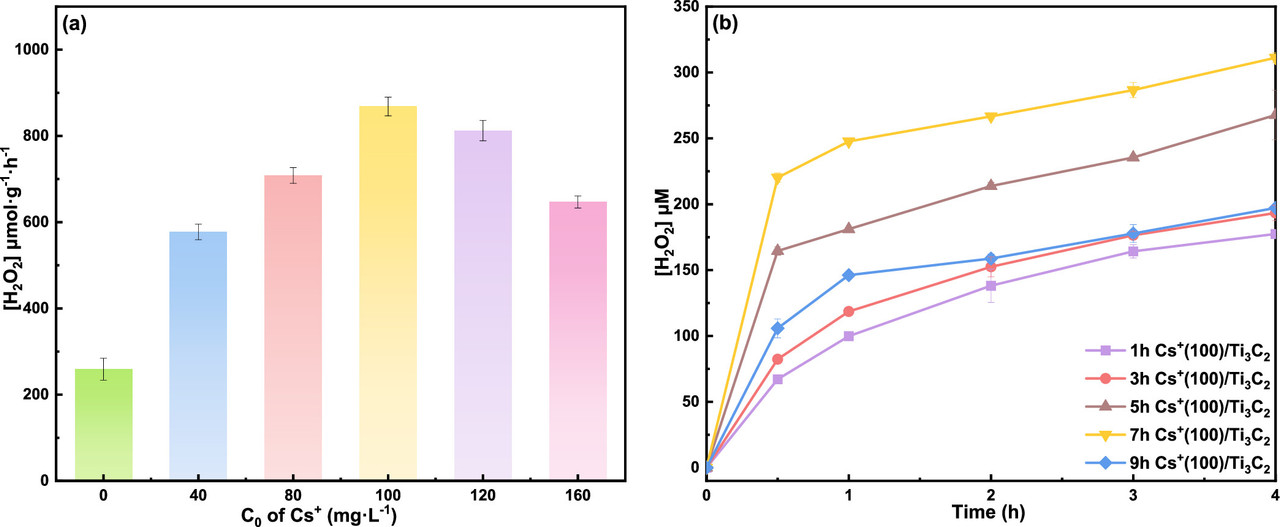
The caption:
Figure 8. (a) H2O2 productivity over Ti3C2 after 0.5 h of Cs+ adsorption with different initial concentrations (conditions: 300 W Xe lamp, 0.025 g photocatalyst, 50 mL seawater, 0 °C, 0.5 h); (b) time-dependent photocatalytic H2O2 production over Cs+(100)/Ti3C2 under different adsorption times for Cs+ (conditions: 300 W Xe lamp, 0.025 g photocatalyst, 50 mL seawater, 0 °C).
From the conclusion:
I have always argued that all components of used nuclear fuel are valuable. This supports that contention.
Have a nice day tomorrow. I trust your Thanksgiving preparations are going well.
Inkey
(467 posts)Thanks for a great in detail description what is happening in chemistry.
Best wishes to you this upcoming holiday season to you and your family !!!